Simultaneous estimation and validation of analgesic and antipyretic drugs in combination from solid dosage form by RP-HPLC
Keywords:
Tramadol hydrochloride, Analgesic, Antipyretic, RP-HPLC, AcetaminophenAbstract
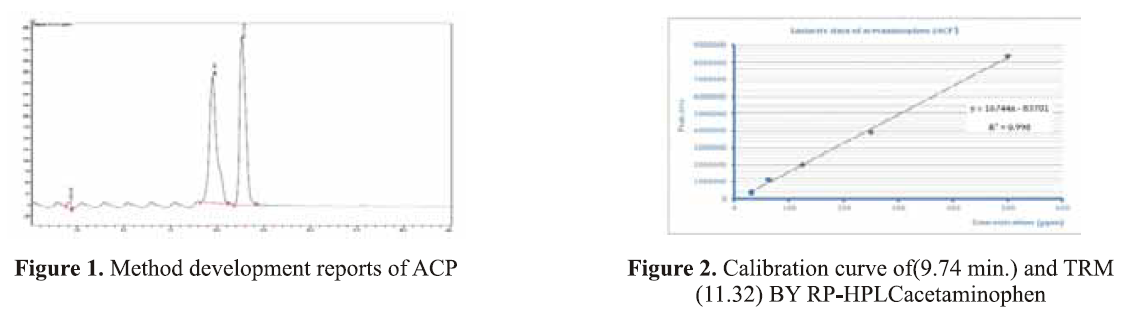
To develop the RP-HPLC method for simultaneous estimation of analgesic and antipyretic drugs in combination from solid dosage form by RP-HPLCmethod.To validate the developed RP-HPLC method as per ICH guidelines. Tramadol hydrochloride is a centrally actinganalgesic. HPLC is a chromatographic technique that can separate a mixture of compounds and is used in biochemistry and analytical chemistry to identify, quantify and purify the individual components of the mixture. System suitabilitytest in that Capacityfactor, Tailingfactor, Resolution, Selectivity, Separationfactor, Theoreticalplates, Regression co efficient, STD for intercept, LOQ, LOD, Repeatability, Precision studies, Linearity/Calibrationstudies, Robustness, Force degradation/Stability indicatingstudies, Specificity, Drug recovery/accuracy studies. The system suitability test performed for acetaminophen and tramadol has achieved all guideline criteria; including, tailing factor (1), separation factors (a), theoretical plates (N), capacity factor(k'), resolution (R) and RSD (%) values as per the obligatory requirements ofICH and USFDA.The validated stress degradation studies under thermal, oxidative, alkali and acid ascertained no possible degradation products developed for tramadol but as observed acetaminophen was slightly degraded indilute HCl (0.lNHCl) and peroxide (3%H2O2). This developed method by reverse phase liquid chromatography (HPLC) can be used for routine analysis of simultaneous estimation of acetaminophen and tramadol for its high precision, reproducibility, and accuracy for any marketed formulation containing either or both of acetaminophen andtramadol.