RP-HPLC Method for The Estimation of Amifostine in Pharmaceutical Dosage Forms
Keywords:
Amifostine, RP-HPLC, Acetonitrile, MethanolAbstract
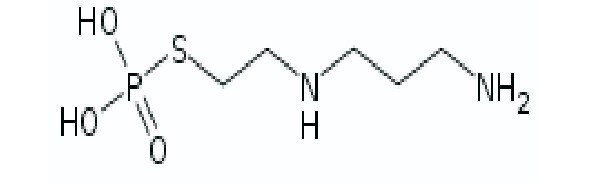
A simple and precise RP-HPLC method was developed and validated for the determination of Amifostine in pharmaceutical dosage forms. Chromatography was carried out using Hypersil C 150 x 4.6 18 mm, 5, ACN:Methanol (70:30) as the mobile phase at a flow rate 1.2 ml/min. The analyte was monitored using UV detector at 246 nm. The retention time of the drug was 3.34 min for Amifostine. The proposed method was found to have linearity in the concentration range of 25–150 μg/ml with correlation coefficient of r²=0.9999. The developed method has been statistically validated and found simple and accurate. The mean recoveries obtained for Amifostine were in the range 100.6-101.9%. Due to its simplicity, rapidness, high precision and accuracy of the proposed method it may be used for determining Amifostine in bulk and dosage forms.