Indole–Pyrazole Conjugates: Synthetic Approaches And Therapeutic Potential
DOI:
https://doi.org/10.5530/ajphs.2025.15.81Keywords:
Indole-pyrazole conjugates, Synthetic approaches, Pharmacological activities, Anticancer agents, Anti-inflammatory, Drug designAbstract
Indole-pyrazole hybrids represent a unique class of molecular frameworks that merge two pharmacologically privileged scaffolds into a single entity, offering broad-spectrum therapeutic potential. The indole nucleus, frequently found in bioactive natural products and neurotransmitters, provides excellent bioavailability and biological compatibility, while the pyrazole ring contributes electronic richness, stability, and versatile reactivity. Their conjugation has yielded derivatives with enhanced potency, selectivity, and multi-target activity. This review highlights recent advances in synthetic methodologies, ranging from classical approaches, such as the Fischer and Bartoli reactions, to environmentally benign strategies, including one-pot, microwave-assisted, and solvent-free techniques. Structural modifications at key positions of both indole and pyrazole rings, as well as linker variations, are critically examined for their role in optimizing pharmacological properties. A comprehensive account of the pharmacological activities of indole–pyrazole conjugates is presented, with significant evidence of anti-inflammatory, analgesic, antioxidant, antimicrobial, antidiabetic, and anticancer potential, supported by both in vitro and in vivo studies. Collectively, the indole–pyrazole framework offers a fertile platform for rational drug design, and ongoing research integrating synthetic innovation with biological evaluation may pave the way for the development of clinically relevant therapeutics.
References
Ali, A., Ali Shah, M. I., Fu, C., Hussain, Z., Qureshi, M. N., Farman, S., Parveen, Z., Zada, A., & Nay, S. (2023). Dihydropyrazole derivatives act as potent α-amylase inhibitors and free radical scavengers: Synthesis, bioactivity evaluation, structure–activity relationship, ADMET, and molecular docking studies. ACS Omega, 8(2), 20412–20422. https://doi.org/10.1021/acsomega.3c00529
Al-Mulla, A. J. D. P. C. (2017). A review: Biological importance of heterocyclic compounds. Der Pharma Chemica, 9, 141–147.
Anastas, P., & Eghbali, N. (2010). Green chemistry: Principles and practice. Chemical Society Reviews, 39, 301–312. The Royal Society of Chemistry. https://doi.org/10.1039/B918763B
Atta, E. M., Mohamed, N. H., & Abdelgawad, A. A. M. (2017). Antioxidants: An overview on the natural and synthetic types. European Chemical Bulletin, 6(8), 365–375.
Babijczuk, K., Wawrzyniak, K., & Warzajtis, B. (2025). Indole–pyrazole hybrids: Synthesis, structure, and assessment of their hemolytic and cytoprotective properties. International Journal of Molecular Sciences, 26(18):9018. https://doi.org/10.3390/ijms26189018
Bedlovičová, Z. (2022). Green synthesis of silver nanoparticles using actinomycetes. In Green synthesis of silver nanomaterials. Elsevier. https://doi.org/10.1016/B978-0-12-824508-8.00001-0
Betcke, I., Götzinger, A. C., Kornet, M. M., & Müller, T. J. (2024). Multicomponent syntheses of pyrazoles via (3+2)-cyclocondensation and (3+2)-cycloaddition key steps. Beilstein Journal of Organic Chemistry, 20, 2024-2077. https://doi.org/10.3762/bjoc.20.178
Black, D. S. C., Gatehouse, B., Theobald, F., & Wong, L. (1980). Investigation of the Bischler indole synthesis from 3,5-dimethoxyaniline. Australian Journal of Chemistry, 33(2), 343–350. https://doi.org/10.1071/CH9800343
Dalpozzo, R., & Bartoli, G. (2005). Bartoli indole synthesis. Current Organic Chemistry, 9(2), 163–178. https://doi.org/10.2174/1385272053369204
Ebenezer, O., Shapi, M., & Tuszynski, J. A. (2022). A review of the recent development in the synthesis and biological evaluations of pyrazole derivatives. Biomedicines, 10(5), 1124. https://doi.org/10.3390/biomedicines10051124
Fabitha, K., Chandrakanth, M., Pramod, R. N., Arya, C., Li, Y., & Banothu, J. (2022). Recent developments in the synthesis of indole‐pyrazole hybrids. ChemistrySelect, 7, e202201064. https://doi.org/10.1002/slct.202201064
Gharge, S., & Alegaon, S. G. (2024). Recent studies of nitrogen and sulfur containing heterocyclic analogues as novel antidiabetic agents: A review. Chemistry & Biodiversity, 21(2), e202301738. https://doi.org/10.1002/cbdv.202301738
Gribble, G. W. (1994). Recent developments in indole ring synthesis—Methodology and applications. Contemporary Organic Synthesis, 1, 145–172. https://doi.org/10.1039/CO9940100145
Grivennikov, S. I., Greten, F. R., & Karin, M. (2010). Immunity, inflammation, and cancer. Cell, 140(6), 883–899. https://doi.org/10.1016/j.cell.2010.01.025
Hassan, A. S., Moustafa, G. O., Awad, H. M., Nossier, E. S., & Mady, M. F. (2021). Design, synthesis, anticancer evaluation, enzymatic assays, and a molecular modeling study of novel pyrazole–indole hybrids. ACS Omega, 6(18), 12361–12374. https://doi.org/10.1021/acsomega.1c01604
Hassan, Z. Q. M., & Abdulkarim, M. G. (2023). Synthesis, characterization, and biological activity evaluation of chalcones and pyrazole derivatives derived from indole. Al-Yarmouk Journal, 21(3), 42–52.
Hawash, M., Ergun, S. G., Kahraman, D. C., Olgac, A., Hamel, E., Cetin-Atalay, R., & Baytas, S. N. (2023). Novel indole-pyrazole hybrids as potential tubulin-targeting agents; Synthesis, antiproliferative evaluation, and molecular modeling studies. Journal of Molecular Structure, 5,1285-135477. https://doi.org/10.1016/j.molstruc.2023.135477
Jiang, B., & Gu, X.-H. (2000). Syntheses and cytotoxicity evaluation of bis(indolyl) thiazole, bis(indolyl) pyrazinone and bis(indolyl) pyrazine: Analogues of cytotoxic marine bis(indole) alkaloid. Bioorganic & Medicinal Chemistry, 8(2), 363–371. https://doi.org/10.1016/S0968-0896(99)00290-4
Kabir, E., & Uzzaman, M. J. (2022). A review on biological and medicinal impact of heterocyclic compounds. Reactive and Inorganic Chemistry, 4, 100606. https://doi.org/10.1016/j.rechem.2022.100606
Kaur, S., Kumari, P., Singh, G., Bhatti, R., & Singh, P. (2018). Design and synthesis of aza-/oxa heterocycle-based conjugates as novel anti-inflammatory agents targeting cyclooxygenase-2. ACS Omega, 3(5), 5825–5845. https://doi.org/10.1021/acsomega.8b00445
Kumar, D., Kumar, R. R., Pathania, S., Singh, P. K., Kalra, S., & Kumar, B. (2021). Investigation of indole functionalized pyrazoles and oxadiazoles as anti-inflammatory agents: Synthesis, in vivo, in vitro and in silico analysis. Bioorganic Chemistry, 114, 105068. https://doi.org/10.1016/j.bioorg.2021.105068
Kumar, D., Sharma, S., Kalra, S., Singh, G., Monga, V., & Kumar, B. (2020). Medicinal perspective of indole derivatives: Recent developments and structure-activity relationship studies. Current Drug Targets, 21(9), 864–891. https://doi.org/10.2174/1389450121666200310115327
Majola, S., Sabela, M., Gengan, R., & Makhanya, T. J. (2024). A facile one‐pot synthesis of new fused indole pyrazole derivatives and their anticancer and antidiabetic activities. Chemistry, 9(48), e202404759. https://doi.org/10.1002/slct.202404759
Medzhitov, R. (2010). Inflammation 2010: New adventures of an old flame. Cell, 140(6), 771–776. https://doi.org/10.1016/j.cell.2010.03.006
Mohamed, M. F., Ibrahim, N. S., Saddiq, A. A., & Abdelhamid, I. A. (2023). Novel 3-(pyrazol-4-yl)-2-(1H-indole-3-carbonyl) acrylonitrile derivatives induce intrinsic and extrinsic apoptotic death mediated P53 in HCT116 colon carcinoma. Scientific Reports, 13(1), 22486. https://doi.org/10.1038/s41598-023-48494-7
Mondal, D., Kalar, P. L., Kori, S., Gayen, S., & Das, K. (2020). Recent developments on synthesis of indole derivatives through green approaches and their pharmaceutical applications. Current Organic Chemistry, 24(22), 2665–2693. https://doi.org/10.2174/1385272824999201111203812
Nehra, B., Kumar, M., Chawla, V., & Chawla, P. A. (2025). Current progress in synthetic and medicinal chemistry of pyrazole hybrids as potent anticancer agents with SAR studies. Future Journal of Pharmaceutical Sciences, 11(1), 75. https://doi.org/10.1186/s43094-025-00821-7
Naglah, A. M., Almehizia, A. A., Ghazwani, M., Al-Wasidi, A. S., Naglah, A. A., Aboulthana, W. M., & Hassan, A. S. (2025). In vitro enzymatic and computational assessments of pyrazole–isatin and pyrazole–indole conjugates as antidiabetic, anti-arthritic, and anti-inflammatory agents. Pharmaceutics, 17(3), 293. https://doi.org/10.3390/pharmaceutics17030293
Patel, V. K., & Rajak, H. (2016). Synthesis, biological evaluation and molecular docking studies of 2-amino-3,4,5-trimethoxyaroylindole derivatives as novel anticancer agents. Bioorganic & Medicinal Chemistry Letters, 26(9), 2115–2118. https://doi.org/10.1016/j.bmcl.2016.03.081
Saini, M. S., Kumar, A., Dwivedi, J., & Singh, R. (2013). A review: Biological significances of heterocyclic compounds. International Journal of Pharmaceutical Sciences and Research, 4(3), 66–77.
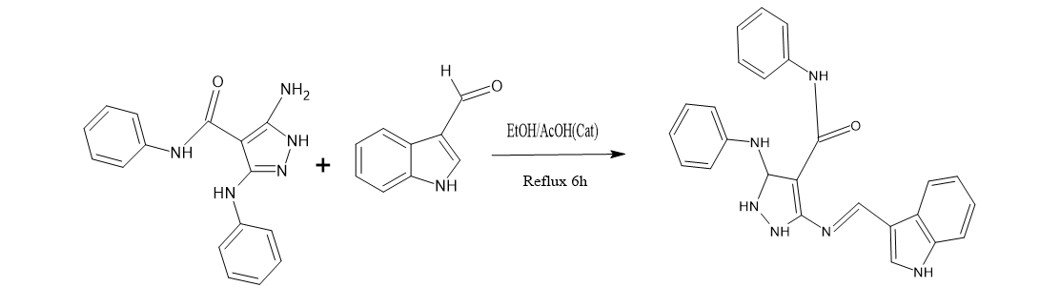
Sapkota, K. R., & Faizi, M. S. H. (2025). Synthesis and spectral characterization of a novel indole-pyrazole-based Schiff base. Oodbodhan, 8, 129–134. https://doi.org/10.3126/oodbodhan.v8i1.81259
Sharath, V., Kumar, H. V., & Naik, N. (2013). Synthesis of novel indole-based scaffolds holding pyrazole ring as anti-inflammatory and antioxidant agents. Journal of Pharmacy Research, 6(7), 785–790. https://doi.org/10.1016/j.jopr.2013.07.002
Soltys, L., Olkhovyy, O., Tatarchuk, T., & Naushad, M. (2021). Green synthesis of metal and metal oxide nanoparticles: Principles of green chemistry and raw materials. Magnetochemistry, 7(11), 145. https://doi.org/10.3390/magnetochemistry7110145
Taber, D. F., & Tirunahari, P. K. (2011). Indole synthesis: A review and proposed classification. Tetrahedron, 67(38), 7195–7210. https://doi.org/10.1016/j.tet.2011.06.040
Takeuchi, O., & Akira, S. (2010). Pattern recognition receptors and inflammation. Cell, 140(6), 805–820. https://doi.org/10.1016/j.cell.2010.01.022
Varga, L., Nagy, T., Dormán, G., Kálmán, F., Ürge, L., & Darvas, F. (2006). Solution-phase parallel synthesis of a pyridinium pyrazol-3-olate inner salt library using a three-component reaction. Journal of Combinatorial Chemistry, 8(3), 338–343. https://doi.org/10.1021/cc0501514
Yang, J.-H., Han, Y.-S., Park, M., Park, T., Hwang, S.-J., & Choy, J.-H. (2007). New inorganic-based drug delivery system of indole-3-acetic acid-layered metal hydroxide nanohybrids with controlled release rate. Chemistry of Materials, 19(10), 2679–2685. https://doi.org/10.1021/cm070259h
Zhang, F., Zhao, Y., Sun, L., Ding, L., Gu, Y., & Gong, P. (2011). Synthesis and anti-tumor activity of 2-amino-3-cyano-6-(1H-indol-3-yl)-4-phenylpyridine derivatives in vitro. European Journal of Medicinal Chemistry, 46(7), 3149-3157. https://doi.org/10.1016/j.ejmech.2011.03.055