Development and Validation of Spectrophotometric Methods for Quantitative Estimation of DiloxanideFuroate in Presence of Its Alkali-induced Degradation Product: A Comparative Study
Keywords:
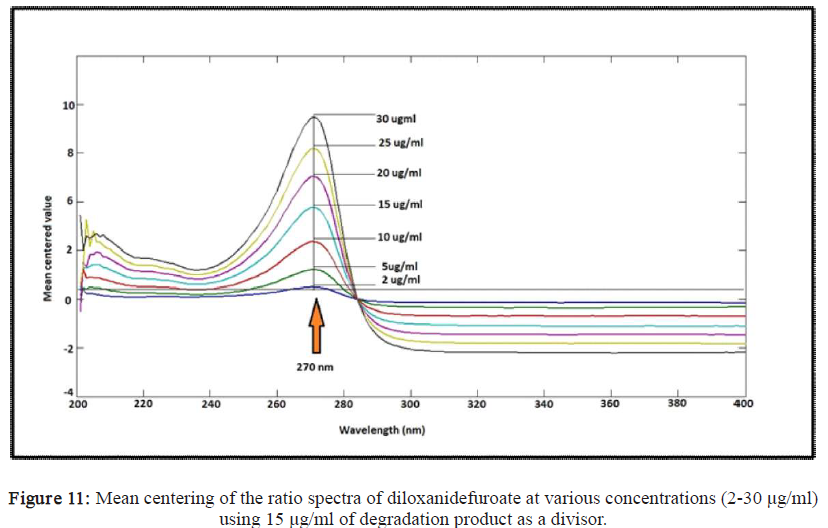
Diloxanidefuroate, alkali-induced degradation, ratio difference, mean centering, SpectrophotometricAbstract
This study aimed to develop and validate two simple, accurate, selective, reproducible and sensitive spectrophotometric methods for the determination of diloxanidefuroate in the presence of its alkali-induced degradation product without preliminary separation. (A) ratio difference spectrophotometry method, where the peak amplitudes of ratio spectra were measured at270nm and 240 nm, (B)mean centering method, where the peak amplitudes were measured at270nm. All methods were applied in the range of (2-30µg mL⁻¹). These methods were validated according toInternational Conference on Harmonization (ICH) guidelines and successfully applied for determination of diloxanidefuroate in Furamebe® tablets. The obtained results were statistically compared with those of the reported method by applying t-test and F-test at 95% confidence level and no significant difference was observed regarding accuracy and precision. The proposed methods are simple, rapid, economic, accurate and precise to determine diloxanidefuroate in the presence of its alkali-induced degradation product without previous separation steps.