Analytical method development and validation for the estimation of Cinnarizine by RP-HPLC in bulk and pharmaceutical dosage forms
Keywords:
Cinnarizine, RP-HPLC, Linearity, Dosage formAbstract
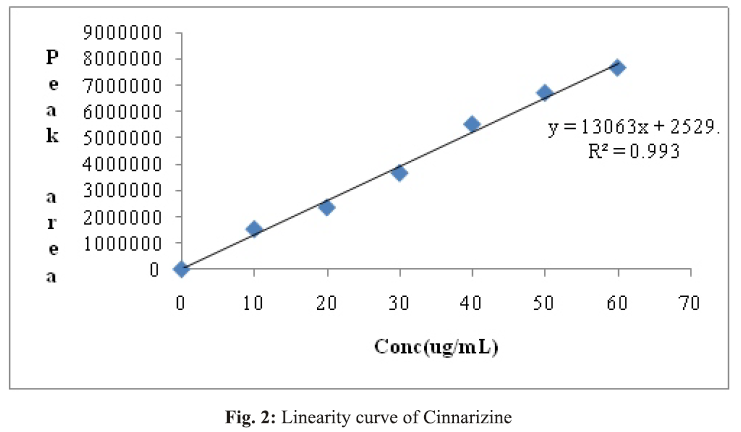
A simple, sensitive, accurate and precise RP-HPLC method was developed for the determination of Cinnarizine in bulk and pharmaceutical dosage forms. The method was developed by using ODS C18 column (250 × 4.6 mm, 5 μ) and the mobile phase composed of acetonitrile: buffer (0.1% ortho -phosphoric acid) in the ratio of 80:20 v/v. The buffer pH was adjusted to 3. The retention time for Cinnarizine was found to be 4.427 min. Linearity range for Cinnarizine was found to be 10–60 μg/mL and the regression equation was found to be y = 130638x + 2529.6. %RSD for intra- and inter-day precision was found to be 0.52% and 0.29%. Average mean recovery was found to be 99.06%. LOD and LOQ values obtained for Cinnarizine were found to be 1.27 and 3.25 μg/mL respectively. The results are analyzed statistically and are found to be satisfactory. Hence this method can be successfully employed for analysis of Cinnarizine in tablet dosage form.