Pyrazole-pyrazine conjugates as potential therapeutic agents: design,synthesis and bioactivity
DOI:
https://doi.org/10.5530/ajphs.2025.15.84Keywords:
Pyrazole, Pyrazine, Synthetic strategies, SAR, Heterocyclic compounds, Pyrazine-Pyrazole hybridsAbstract
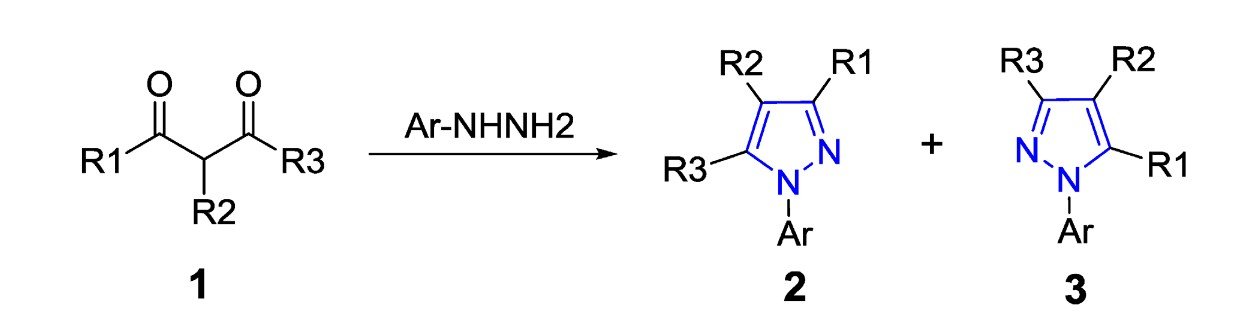
Heterocyclic compounds linked with a pharmacophore exhibit better biological activity. The pyrazole molecule linked to pyrazine-2-carbohydrazide derivatives represents a novel class of compounds with various therapeutic potential. This review mainly focused on the design and synthesis of dual heterocyclic scaffolds, including conventional and green synthetic strategies. It also includes ADMET profiling, which helps predict pharmacokinetic properties, target interactions, etc., as well as structure-activity relationships. Comprehensive biological activities, including antimicrobial, anticancer, anti-inflammatory, and anticonvulsant, of these dual heterocyclic compounds are also included. Through structure-activity relationships, the influence of different functional groups on activity can be determined. This review emphasizes the significant therapeutic promise of pyrazole-pyrazine conjugates and proposes future research pathways.
References
Abu-Zaied, M. A., Elgemeie, G. H., & Mohamed-Ezzat, R. A. (2024). Novel Acrylamide-Pyrazole Conjugates: Design, Synthesis and Antimicrobial Evaluation. Egyptian Journal of Chemistry, 67(13), 529-536. https://doi.org/10.21608/ejchem.2024.258180.9074
Akiyama, T., Enomoto, Y., & Shibamoto, T. (1978). A new method of pyrazine synthesis for flavor use. Journal of Agricultural and Food Chemistry, 26(5), 1176-1179.
Aljamali, N. M. (2014). Review in cyclic compounds with heteroatom. Asian Journal of Research in Chemistry, 7(11), 975-1006.
Arora, P., Arora, V., Lamba, H., & Wadhwa, D. (2012). Importance of heterocyclic chemistry: A review. International Journal of Pharmaceutical Sciences and Research, 3(9), 2947.
Barlin, G. B. (2009). The Pyrazines, Volume 41 (Vol. 41). John Wiley & Sons.
Bhandari, S., Tripathi, A. C., & Saraf, S. K. (2013). Novel 2-pyrazoline derivatives as potential anticonvulsant agents. Medicinal Chemistry Research, 22(11), 5290-5296. https://doi.org/10.1007/s00044-013-0530-7
Chen, G.-Q., Guo, H.-Y., Quan, Z.-S., Shen, Q.-K., Li, X., & Luan, T. (2023). Natural products–pyrazine hybrids: a review of developments in medicinal chemistry. Molecules, 28(21), 7440. https://doi.org/10.3390/molecules28217440
Correia, M. A., & Ortiz de Montellano, P. R. (2005). Inhibition of cytochrome P450 enzymes. Cytochrome P, 450, 247-322. https://doi.org/10.1007/978-1-4757-2391-5_9
Das, B., Baidya, A. T., Mathew, A. T., Yadav, A. K., & Kumar, R. (2022). Structural modification aimed for improving solubility of lead compounds in early phase drug discovery. Bioorganic & Medicinal Chemistry, 56, 116614. https://doi.org/10.1016/j.bmc.2022.116614
Duffin, G. (1964). The quaternization of heterocyclic compounds. In Advances in Heterocyclic Chemistry (Vol. 3, pp. 1-56). Elsevier. https://doi.org/10.1016/S0065-2725(08)60540-1
Ebenezer, O., Shapi, M., & Tuszynski, J. A. (2022). A review of the recent development in the synthesis and biological evaluations of pyrazole derivatives. Biomedicines, 10(5), 1124. https://doi.org/10.3390/biomedicines10051124
El-Sabbagh, O. I., Baraka, M. M., Ibrahim, S. M., Pannecouque, C., Andrei, G., Snoeck, R., Balzarini, J., & Rashad, A. A. (2009). Synthesis and antiviral activity of new pyrazole and thiazole derivatives. European Journal of Medicinal Chemistry, 44(9), 3746-3753. https://doi.org/10.1016/j.ejmech.2009.03.038
Elderfield, R. (1950). Heterocyclic compounds. John Wiley & Sons, Inc.
Faisal, M., Saeed, A., Hussain, S., Dar, P., & Larik, F. A. (2019). Recent developments in synthetic chemistry and biological activities of pyrazole derivatives. Journal of Chemical Sciences, 131(8), 70. https://doi.org/10.1007/s12039-019-1646-1
Farghaly, A.-R., Esmail, S., Abdel-Zaher, A., Abdel-Hafez, A., & El-Kashef, H. (2014). Synthesis and anticonvulsant activity of some new pyrazolo [3, 4-b] pyrazines and related heterocycles. Bioorganic & Medicinal Chemistry, 22(7), 2166-2175. https://doi.org/10.1016/j.bmc.2014.02.019
Feng, F., Xu, D.-Q., Yue, S.-J., Chen, Y.-Y., & Tang, Y.-P. (2024). Neuroprotection by tetramethylpyrazine and its synthesized analogues for central nervous system diseases: A review. Molecular Biology Reports, 51(1), 159. https://doi.org/10.1007/s11033-023-09068-y
Grimmett, M., Lim, K. R., & Weavers, R. (1979). The N-Alkylation and N-arylation of unsymmetrical pyrazoles. Australian Journal of Chemistry, 32(10), 2203-2213. https://doi.org/10.1071/CH9792203
Hm, A. B., & Dubey, S. (2024). Fundamental chemistry of n-heterocycles: pyrrole and pyrazole chemistry, imidazole chemistry, triazole chemistry advances in heterocyclic ring systems. Paving the Path to Discoveries and Unlocking the Secrets of N-Heterocycles, Cambridge Scholars Publishing. 64.
Hosamani, K. R., K, H., Pal, R., Matada, G. S. P., B, K., I, A., & Aishwarya, N. V. S. S. (2024). Pyrazole, Pyrazoline, and Fused Pyrazole Derivatives: New Horizons in EGFR‐Targeted Anticancer Agents. Chemistry & Biodiversity, 21(11), e202400880. https://doi.org/10.1002/cbdv.202400880
Hou, W., Dai, W., Huang, H., Liu, S.-L., Liu, J., Huang, L.-J., Huang, X.-H., Zeng, J.-L., Gan, Z.-W., & Zhang, Z.-Y. (2023). Pharmacological activity and mechanism of pyrazines. European Journal of Medicinal Chemistry, 258, 115544. https://doi.org/10.1016/j.ejmech.2023.115544
Juhas, M., & Zitko, J. (2020). Molecular interactions of pyrazine-based compounds to proteins. Journal of Medicinal Chemistry, 63(17), 8901-8916. https://doi.org/10.1021/acs.jmedchem.9b02021
Karrouchi, K., Radi, S., Ramli, Y., Taoufik, J., Mabkhot, Y. N., Al-Aizari, F. A., & Ansar, M. H. (2018). Synthesis and pharmacological activities of pyrazole derivatives: A review. Molecules, 23(1), 134. https://doi.org/10.3390/molecules23010134
Kitawat, B. S., & Singh, M. (2014). Synthesis, characterization, antibacterial, antioxidant, DNA binding and SAR study of a novel pyrazine moiety bearing 2-pyrazoline derivatives. New Journal of Chemistry, 38(9), 4290-4299. https://doi.org/10.1039/C4NJ00594E
Knorr, L. (1883). Einwirkung von acetessigester auf phenylhydrazin. Berichte der deutschen chemischen Gesellschaft, 16(2), 2597-2599. https://doi.org/10.1002/cber.188301602194
Kumar, B., Smita, K., Cumbal, L., & Debut, A. (2017). Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi Journal of Biological Sciences, 24(1), 45-50. https://doi.org/10.1016/j.sjbs.2015.09.006
Li, G., Cheng, Y., Han, C., Song, C., Huang, N., & Du, Y. (2022). Pyrazole-containing pharmaceuticals: target, pharmacological activity, and their SAR studies. RSC Medicinal Chemistry, 13(11), 1300-1321. https://doi.org/10.1039/D2MD00206J
Maklad, N. S. (2012). Chichibabin Amination Reaction. ChemInform, 43(19), no-no.
Marek, R., & Lycka, A. (2002). 15N NMR spectroscopy in structural analysis. Current Organic Chemistry, 6(1), 35-66. https://doi.org/10.2174/1385272023374643
Marinescu, M. (2021). Synthesis of antimicrobial benzimidazole–pyrazole compounds and their biological activities. Antibiotics, 10(8), 1002. https://doi.org/10.3390/antibiotics10081002
Ogryzek, M., Chylewska, A., Królicka, A., Banasiuk, R., Turecka, K., Lesiak, D., Nidzworski, D., & Makowski, M. (2016). Coordination chemistry of pyrazine derivatives analogues of PZA: Design, synthesis, characterization and biological activity. RSC Advances, 6(57), 52009-52025. https://doi.org/10.1039/C6RA03068H
Özdemir, A., Turan-Zitouni, G., Kaplancikli, Z. A., & Tunali, Y. (2009). Synthesis and biological activities of new hydrazide derivatives. Journal of Enzyme Inhibition and Medicinal Chemistry, 24(3), 825-831. https://doi.org/10.1080/14756360802399712
Pathak, S., Agrawal, N., & Gaur, S. (2024). A review on diverse biological activity of heterocyclic nucleus pyrazine and its derivatives: A key for the researchers. Letters in Organic Chemistry, 21(4), 351-361. https://doi.org/10.2174/0115701786273932230927062616
Sahu, B., Sahu, R., Gidwani, B., & Mishra, A. (2024). Pyrrole: An Essential Framework in the Development of Therapeutic Agents and Insightful Analysis of Structure‐Active Relationships. ChemistrySelect, 9(31), e202401604. https://doi.org/10.1002/slct.202401604
Seliem, I. A., Girgis, A. S., Moatasim, Y., Kandeil, A., Mostafa, A., Ali, M. A., Bekheit, M. S., & Panda, S. S. (2021). New pyrazine conjugates: synthesis, computational studies, and antiviral properties against SARS‐CoV‐2. ChemMedChem, 16(22), 3418-3427. https://doi.org/10.1002/cmdc.202100476
Singh, G., Chandra, P., & Sachan, N. (2020). Chemistry and pharmacological activities of pyrazole and pyrazole derivatives: a review. International Journal of Pharmaceutical Sciences Review and Research, 65(1), 201-214. https://doi.org/10.47583/ijpsrr.2020.v65i01.030
Singh, I., Luxami, V., & Paul, K. (2020). Synthesis, cytotoxicity, pharmacokinetic profile, binding with DNA and BSA of new imidazo [1, 2-a] pyrazine-benzo [d] imidazol-5-yl hybrids. Scientific Reports, 10(1), 6534. https://doi.org/10.1038/s41598-020-63605-4
Tınmaz, F., İlhan, İ. Ö., & Akkoç, S. (2020). Preparation and Properties of Some New Pyrazole Derivatives. Organic Preparations and Procedures International, 53(1), 89-94. https://doi.org/10.1080/00304948.2020.1846449
Vitaku, E., Smith, D. T., & Njardarson, J. T. (2014). Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals: miniperspective. Journal of Medicinal Chemistry, 57(24), 10257-10274. https://doi.org/10.1021/jm501100b
Wren, S. W., Vogelhuber, K. M., Garver, J. M., Kato, S., Sheps, L., Bierbaum, V. M., & Lineberger, W. C. (2012). C–H bond strengths and acidities in aromatic systems: Effects of nitrogen incorporation in mono-, di-, and triazines. Journal of the American Chemical Society, 134(15), 6584-6595. https://doi.org/10.1021/ja209566q
Zang, B., & Wang, L. (2023). Synthesis and protective effect of pyrazole conjugated imidazo [1, 2-a] pyrazine derivatives against acute lung injury in sepsis rats via attenuation of NF-κB, oxidative stress and apoptosis. Acta Pharmaceutica, 73(3), 341-362. https://doi.org/10.2478/acph-2023-0031
Zhang, Y., Wu, C., Zhang, N., Fan, R., Ye, Y., & Xu, J. (2023). Recent advances in the development of pyrazole derivatives as anticancer agents. International Journal of Molecular Sciences, 24(16), 12724. https://doi.org/10.3390/ijms241612724