Are Over-the-Counter Acne Drugs Considered Safe?
DOI:
https://doi.org/10.5530/ajphs.2025.15.76Keywords:
Over-the-Counter, Acne vulgaris, Drugs, Community pharmaciesAbstract
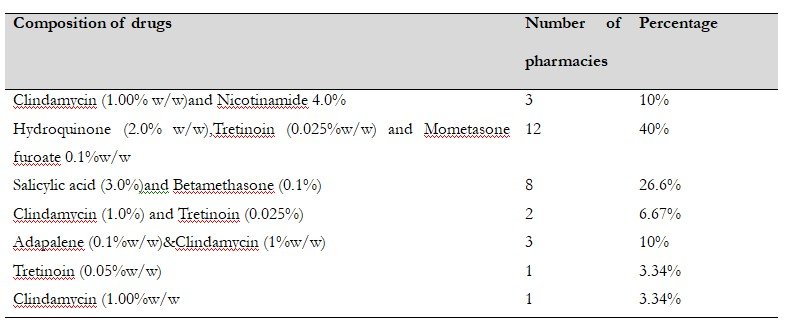
This descriptive observational study was conducted in 30 community pharmacies in Chidambaram, Tamil Nadu, to assess the dispensing of over-the-counter (OTC) acne medications. The most frequently dispensed preparations were hydroquinone + mometasone furoate + tretinoin (40%), followed by salicylic acid + betamethasone (26%). Among the observed combinations, only nicotinamide, salicylic acid, benzoyl peroxide, adapalene, and clindamycin are considered first-line agents in acne management. At the same time, drugs such as hydroquinone, mometasone furoate, tretinoin, and betamethasone are scheduled H drugs requiring physician supervision. The findings highlight the need for a regulated OTC drug list and the removal of inappropriate combination products to ensure safer and more effective acne treatment.