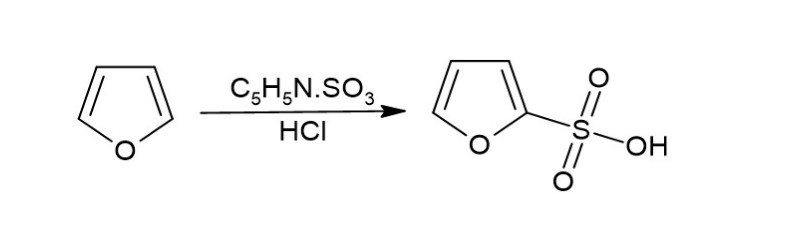
Exploring the role of furan-pyrazole in the treatment of breast cancer: a comprehensive review
DOI:
https://doi.org/10.5530/ajphs.2025.15.79Keywords:
Furan, Pyrazole, Breast cancer, Anti-proliferative, Anti-metastaticAbstract
Breast cancer is one of the most prevalent malignancies among women worldwide, which is one of the major factors leading to morbidity and mortality. These compounds exhibit a range of biological activities, including anti-proliferative, pro-apoptotic, and anti-metastatic properties, making them potential candidates for therapeutic development. Moreover, their structural flexibility enables the development of more effective and selective inhibitors. The mechanism of action and therapeutic potential of furan-pyrazole derivatives may aid in designing breast cancer medicines with higher efficacy and fewer adverse effects. This review examines the role of furan-pyrazole derivatives in breast cancer therapy, focusing on their molecular mechanisms, pharmacological significance, and potential as targeted agents. This review aims to consolidate existing knowledge while highlighting recent advancements in breast cancer research and management with a focus on furan pyrazole compounds
References
1. Bennani FE, Doudach L, Karrouchi K, Rudd CE, Ansar Mh, Faouzi MEA. Design and prediction of novel pyrazole derivatives as potential anti-cancer compounds based on 2D-QSAR study against PC-3, B16F10, K562, MDA-MB-231, A2780, ACHN and NUGC cancer cell lines. Heliyon. 2022;8(8). https://doi.org/10.1016/j.heliyon.2022.e10003
2. Wu Z, Xia F, Lin R. Global burden of cancer and associated risk factors in 204 countries and territories, 1980–2021: a systematic analysis for the GBD 2021. J Hematol Oncol. 2024;17(119). https://doi. org/10.1186/s13045-024-01640-8.
3. Cobb AN, Czaja R, Jorns J, Cortina CS. Breast cancer subtypes: clinicopathologic features and treatment considerations. Curr Breast Cancer Rep. 2024;16(2):150-60. doi: 10.1007/s12609-024-00541-6
4. Sharma GN, Dave R, Sanadya J, Sharma P, Sharma K. Various types and management of breast cancer: an overview. J Adv Pharm Technol Res. 2010;1(2):109-26. DOI: 10.4103/2231-4040.72251
5. Zhang Y, Wu C, Zhang N, Fan R, Ye Y, Xu J. Recent advances in the development of pyrazole derivatives as anticancer agents. Int J Mol Sci. 2023;24(16):12724. https://doi.org/10.3390/ijms241612724
6. Sun Y-S, Zhao Z, Yang Z-N, Xu F, Lu H-J, Zhu Z-Y, et al. Risk factors and preventions of breast cancer. Int J Biol Sci. 2017;13(11):1387-97. doi: 10.7150/ijbs.21635
7. Bansal RK. Heterocyclic chemistry: New Age International; 2020.
8. Katritzky AR, Ramsden CA, Scriven EFV, Taylor RJK. Comprehensive heterocyclic chemistry III. In V1 3-memb. Heterocycl., together with all Fused Syst. contain. a 3-memb. Heterocycl. Ring. V2 4-memb. Heterocycl. together with all Fused Syst. contain. a 4-memb. Heterocycl. Ring. V3 Five-memb. Rings with One Heteroat. together with their Benzo and other Carbocycl.-fused Deriv. V4 Five-memb. Rings with Two Heteroat., each with their Fused Carbocycl. Deriv.. 14 ed. Vol. 1. Elsevier. 2008. p. 1-13718 doi: 10.1016/C2009-1-28335-3
9. Kottke R. Furan derivatives. Kirk‐Othmer Encyclopedia of Chemical Technology. 2000. https://doi.org/10.1002/0471238961.0621180111152020.a01
10. Elderfield R. Heterocyclic compounds: John Wiley & Sons, Inc; 1950.
11. Helmy MT, Sroor FM, Mahrous KF, Mahmoud K, Hassaneen HM, Saleh FM, et al. Anticancer activity of novel 3‐(furan‐2‐yl) pyrazolyl and 3‐(thiophen‐2‐yl) pyrazolyl hybrid chalcones: Synthesis and in vitro studies. Arch Pharm. 2022; 355(3): 2100381. DOI: 10.1002/ardp.202100381
12. Beniwal M, Jain N. Microwave assisted synthesis, characterization and antimicrobial screening of thiazolidin-4-one substituted pyrazole derivatives. Curr Microw Chem. 2019;6(1):44-53. https://doi.org/10.2174/2213335606666190722144956
13. Ashourpour M, Mostafavi-Hosseini F, Amini M, Moghadam ES, Kazerouni F, Arman SY, et al. Pyrazole derivatives induce apoptosis via ROS generation in the triple negative breast cancer cells, MDA-MB-468. Asian Pac J Cancer Prev. 2021; 22(7): 2079. DOI:10.31557/APJCP.2021.22.7.2079
14. Jamwal A, Javed A, Bhardwaj VJJPB. A review on pyrazole derivatives of pharmacological potential. J Pharm BioSci. 2013;3:114-23.
15. Matiadis D, Sagnou M. Pyrazoline hybrids as promising anticancer agents: An up-to-date overview. Int J Mol Sci. 2020; 21(15): 5507. https://doi.org/10.3390/ijms21155507
16. Mor S, Khatri M, Punia R, Nagoria S, Sindhu S. A new insight into the synthesis and biological activities of pyrazole based derivatives. Mini-Rev Org Chem. 2022; 19 (6): 717-78. DOI: 10.2174/1570193X19666220118111614
17. Siddiqui A, Sinha A, Tripathi V, Singh G, Yadav A, Fatima N, et al. Synthetic Strategies And Biological Applications Of Pyrazole: A Review.Res j life sci bioinform pharm chem sci. 2025. DOI: 10.26479/2025.1103.02
18. Zhang Y-L, Li B-Y, Yang R, Xia L-Y, Fan A-L, Chu Y-C, et al. A class of novel tubulin polymerization inhibitors exert effective anti-tumor activity via mitotic catastrophe. Eur J Med Chem. 2019; 163: 896-910. https://doi.org/10.1016/j.ejmech.2018.12.030
19. Manju N, Kalluraya B, Kumar MS. Synthesis, computational and biological study of pyrazole derivatives. J Mol Struct. 2019; 1193: 386-97. https://doi.org/10.1016/j.molstruc.2019.05.049
20. Howard S, Berdini V, Boulstridge JA, Carr MG, Cross DM, Curry J, et al. Fragment-based discovery of the pyrazol-4-yl urea (AT9283), a multitargeted kinase inhibitor with potent aurora kinase activity. J Med Chem. 2009; 52(2): 379-88. https://doi.org/10.1021/jm800984v
21. Sabet R, Hatam G, Emami L, Ataollahi E, Zare F, Zamani L, et al. Pyrazole derivatives as antileishmanial agents: Biological evaluation, molecular docking study, DFT analysis and ADME prediction. Heliyon. 2024;10(23). https://doi.org/10.1016/j.heliyon.2024.e40444
22. Anwar MM, Abd El-Karim SS, Mahmoud AH, Amr AE-GE, Al-Omar MA. A comparative study of the anticancer activity and PARP-1 inhibiting effect of benzofuran–pyrazole scaffold and its nano-sized particles in human breast cancer cells. Molecules. 2019; 24(13): 2413. https://doi.org/10.3390/molecules24132413
23. Mudimela S, Giridharan VV, Janardhan S. Molecular Docking, Synthesis, and Characterization of Furanyl‐Pyrazolyl Acetamide and 2, 4‐Thiazolidinyl‐Furan‐3‐Carboxamide Derivatives as Neuroinflammatory Protective Agents. Chemistry & Biodiversity. 2024; 21(5): e202301260. https://doi.org/10.1002/cbdv.202301260
24. Patel P, Shakya R, Asati V, Kurmi BD, Verma SK, Gupta GD, et al. Furan and benzofuran derivatives as privileged scaffolds as anticancer agents: SAR and docking studies (2010 to till date). J Mol Struct. 2024;1299:137098. https://doi.org/10.1016/j.molstruc.2023.137098
25. Jameson JL, Longo DL. Precision medicine—personalized, problematic, and promising. Obstet Gynecol Surv. 2015; 70(10): 612-4. https://doi.org/10.1097/OGX.0000000000000221
26. O'Dwyer PJ, King SA, Plowman J, Grieshaber CK, Hoth DF, Leyland-Jones B. Pyrazole: preclinical reassessment. Invest New Drugs. 1988;6(4):305-10. https://doi.org/10.1007/BF00173649