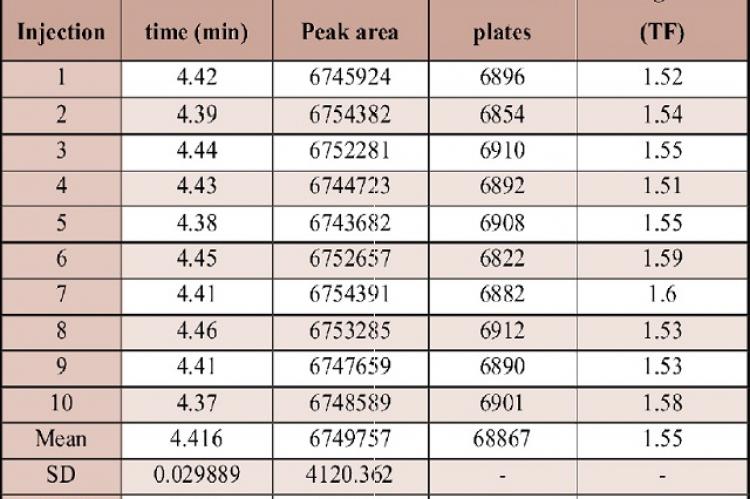
A simple, sensitive, accurate and precise RP-HPLC method was developed for the determination of Cinnarizine in bulk and pharmaceutical dosage forms. The method was developed by using ODS C18 column (250 × 4.6 mm, 5µ) and the mobile phase composed of acetonitrile: buffer (0.1% ortho-phosphoric acid) in the ratio of 80:20v/v. The buffer pH was adjusted to 3. The retention time for Cinnarizine was found to be 4.427 min. Linearity range for Cinnarizine was found to be 10-60 µg/mL and the regression equation was found to be y = 130638x + 2529.6. % RSD for intra- and inter-day precision was found to be 0.52% and 0.29%. Average mean recovery was found to be 99.06%. LOD and LOQ values obtained for Cinnarizine were found to be 1.27 and 3.25 µg/mL respectively. The results are analyzed statistically and are found to be satisfactory. Hence this method can be successfully employed for analysis of Cinnarizine in tablet dosage form.
View:
- PDF (6.03 MB)