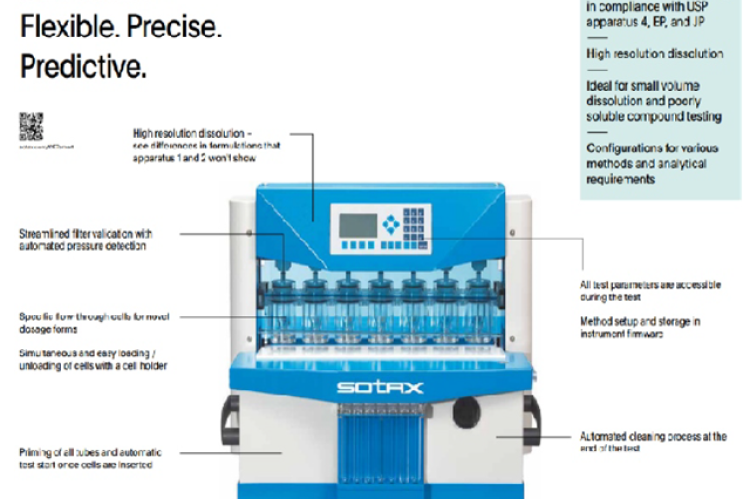
The dissolution apparatus specified by the United States Pharmacopeia (USP) plays a crucial role in pharmaceutical development and quality control, notably in determining the release rate of active pharmaceutical ingredients (APIs) from solid dosage forms. This review provides an extensive account of the history, design concepts, operating parameters, and regulatory issues associated with USP Type 4 dissolution equipment. The key components of dissolution testing, including medium choice, temperature regulation, and sampling methods, are covered in this review. In addition, it focuses on the regulations established by the USP and other global regulatory bodies to guarantee the precision and uniformity of dissolution data produced using Type 4 equipment. Also, it describes the open loop, closed loop configurations, Pump and flow patterns, and types of cells for different dosage forms. Additionally, new developments and technology advancements targeted at enhancing the automation, precision, and efficiency of dissolution testing are discussed. This study provides useful details on the practical application of USP Type 4 dissolving equipment in pharmaceutical development and quality assurance by synthesizing current research and expert perspectives.
View:
- PDF (343.37 KB)