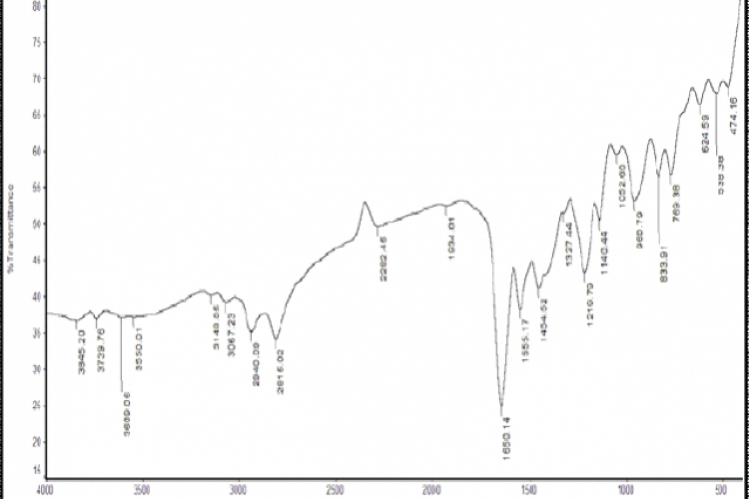
Objective: Accurate and selective reversed phase high performance liquid chromatography (RP-HPLC) method has been developed and validated for determination of brexpiprazole in the presence of its oxidative-induced degradation product. Methods: The developed method was based on the separation of the two components using methanol , water and phosphoric acid (60:40:0.4, by volume) as a mobile phase in isocratic elution mode on ODS SUPELCO C18 (25 cm X 4.6 mm, 5 μm particle size) column at a flow rate of 1 ml min−1 and ultraviolet (UV) detection at 259 nm. Results and discussion: The components were well resolved from each other with significantly different Rt values of 4.41 and 2.59 min for brexpiprazole and its oxidative-induced degradation product, respectively. Conclusion: The method was found to be linear in the range of (20-100 µg/mL). The developed method was validated according to the International Conference on Harmonization (ICH) guidelines demonstrated a good accuracy and precision. The results were statistically compared with those obtained by the reported method, and no significant difference was found.
View:
- PDF (723.23 KB)