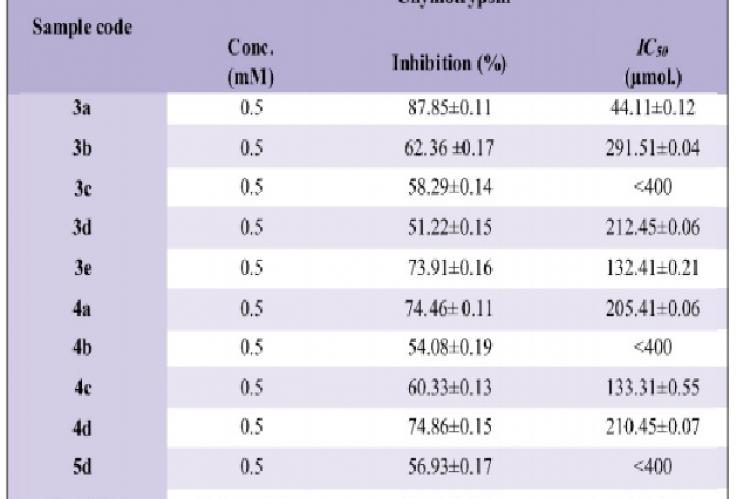
The sole purpose of this research work was to synthesize different O-phenyl-N-aryl carbamates and to evaluate their chymotrypsin activity. A series of O-phenyl-N-aryl carbamates has been synthesized by reacting different disubstituted aromatic amines (2a-e) with phenylchloroformate in basic aqueous media. Further brominated and nitrated carbamates (4a-d, 5d) were synthesized by bromination and nitration of O-phenyl-N-aryl carbamates. Purity of all the compounds was checked by TLC. All these synthesized compounds were characterized by IR, EI-MS, and 1H-NMR and then screened against chymotrypsin enzyme. The screening of the synthesized derivatives revealed their effectiveness against chymotrypsin enzyme. Among these compounds, O-Phenyl-N-(3,5-dimethylphenyl)carbamate (3a) was found to be the most potent inhibitor for chymotrypsin while compound (3d) was found to be the least inhibitor.
View:
- PDF (740.34 KB)