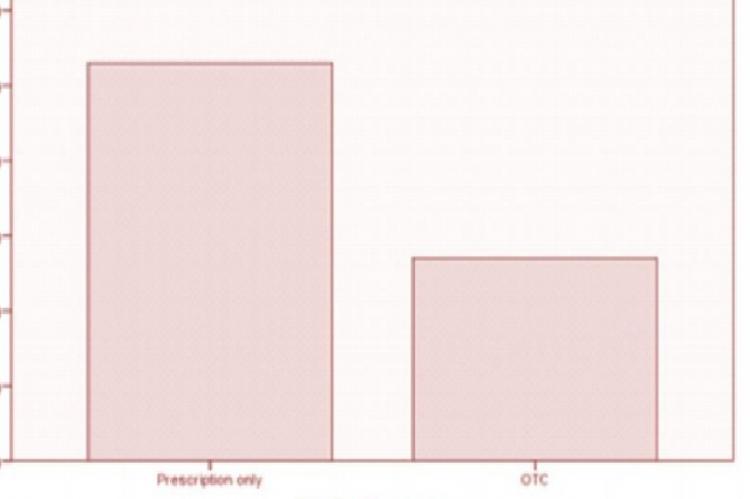
Pharmaceutical package insert (PPI) is a good source of information about the medicines we take. The aim of the present study was to evaluate information written on PPI of products marketed locally in Pakistan. The study comprised of analysis of PPIs (n=80) against 20 criteria extracted from literature. PPI were categorized in prescription and OTC (over-the-counter) drugs and as local and multinational products. Results were summarized as number and frequencies. Chi-square test using 0.05 level of significance was performed to observe the association of medication type and manufacturer type with the PPI meeting the criteria. Most of the PPIs assessed met the criteria set. In some PPIs, there was lacking of information like, direction of drug use, duration of use and drug interactions. It is concluded that information written on PPIs of drugs marketed in Pakistan was incomplete and non-comprehensive. The PPIs should be improved for the safe and effective use of medications.
View:
- PDF (311.02 KB)