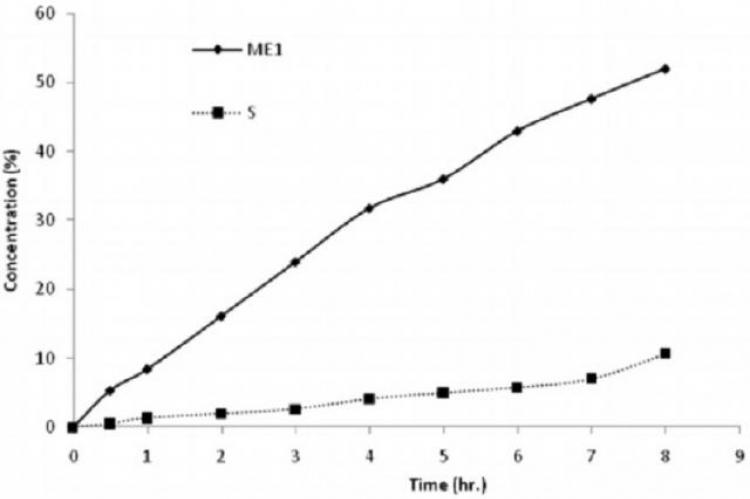
The antidiabetic drug, Repaglinide, was incorporated into a microemulsion and a niosomal carriers for transdermal administration to overcome the drawbacks associated with its oral formula. In the microemulsion formulation, pseudoternary phase diagrams were constructed to obtain the concentration range of the oil, surfactant (S) and co-surfactant (CoS) using three different S/CoS weight ratios. Microemulsion showed spherical particles with mean diameter ranging from 40.60 ± 13.04 to 58.94 ± 0.02 nm and newtonian viscosity ranging from 58.94 ± 0.02 to 111.23 ± 6.32 mPa.S. Compared to the marketed tablet, the release of repaglinide from microemulsion showed a zero order, controlled and continuous pattern. Repaglinide was also encapsulated in niosomal formulations. Niosomes showed vesicle size diameter ranging from 109 ± 6.2 to 263 ± 11.9 nm with an entrapment efficiency of 77.9 ± 5.2 to 98.6 ± 6.3 %. Compared to the oral tablet, release of repaglinide from niosomal vesicles showed a more uniform pattern however, a much slower rate. The microemulsion formulation (ME1) composed of triacetin as oil, Cremophor® RH40 and n-butanol as surfactant and co-surfactant, respectively, together with niosomal formulation (S) prepared using span 60 and cholesterol in ratio 1:1 were selected for the ex-vivo permeation. The permeation rate constant and permeation efficiency were found to be 65.5 2 μg/cm/h and 29.19%, respectively with micro emulsion ME1 2 and 11.42 μg/cm/h and 4.14%, respectively with niosomal preparation S. It was concluded that the microemulsion transdermal delivery systems offered a controlled and more sustained drug release and permeation profiles compared to the niosomal formulations and to the commercial tablet.
View:
- PDF (808.25 KB)