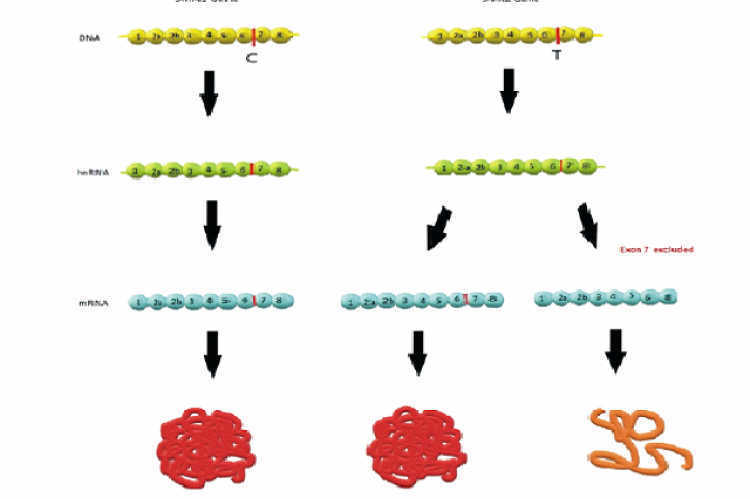
Spinal Muscular Atrophy, a fatal genetic neuromuscular disease is distinguished by progressive muscular weakness , atrophy and paralysis . The 5q SMA is characterized by the degeneration of alpha motor neurons in the ventral horn of the spinal cord. It has a clinical spectrum that ranges from premature death of an infant to a normal life with slightly expressive muscular weakness. Onasmogene Abeparvovec is one of the three FDA approved treatment options for this disease. It is a gene therapy that is non replicating and self complementary in nature . It is an Adeno Associated Virus -9(AAV 9) vector that delivers a completely functioning SMN gene that encodes the SMN protein to the neurons, thus addressing the root cause of SMA by delivering the missing SMN1 gene to the neurons. This drug can enhance the motor functioning of the infants but it cannot reverse any disabilities appeared before the administration of the drug .Although clinical trials have revealed a tremendous information about the drug, it is imperative for these studies to continue to gather knowledge on its long term use.
View:
- PDF (956.48 KB)