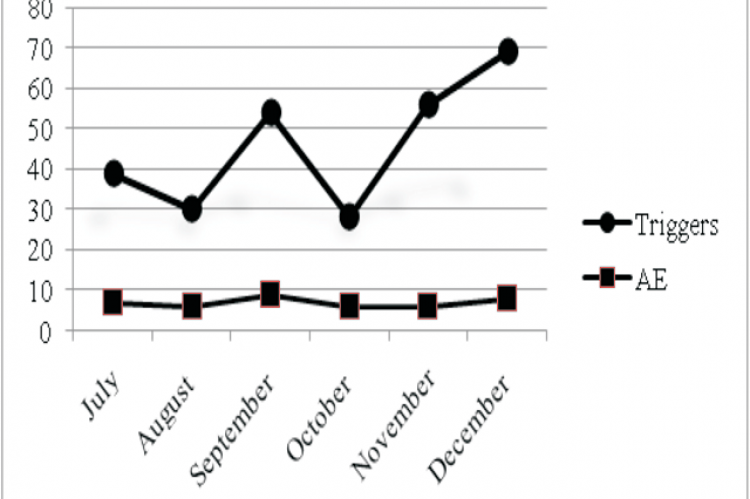
A trigger is defined as an "occurrence, prompt, or flag found on review of the medical record that 'triggers' further investigation to determine the presence or absence of an adverse event". Trigger tool method is a measure of hospital safety programme which helps to assess the level of harm. The present study was aimed to develop a list of triggers to identify adverse events in a surgery department of a tertiary care teaching hospital. The development of trigger tool was carried out in six stages including literature review, expert panel review, three rounds of Delphi panel review and consolidation. Delphi Panel review was found to be suitable for the development of trigger tools. A total of 120 case records were reviewed using the developed tools for a period of six months. The triggers were studied for their presence, frequency, ability to pick up adverse events. The developed trigger list was able to flag 75 case profiles out of 120 reviewed case reports with potential adverse events. AEs were identified in 35% of the reviewed cases. The triggers like transfusion/ use of blood products, repeated request lab assessment were able to identify AEs more frequently. The developed tool of the present study may be suitable for Indian settings if validated further in other centres and will be valuable in enhancing patient safety in surgical settings.
View:
- PDF (1.97 MB)