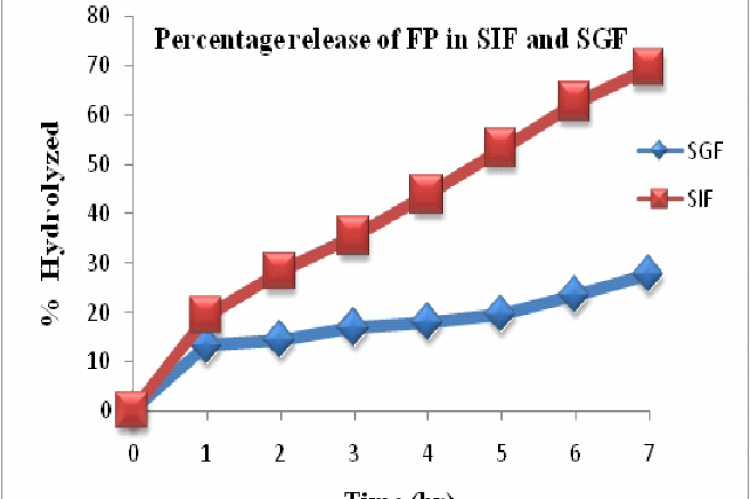
The aim of the present study is to synthesize the aminoacid prodrugs of flurbiprofen and perform the physico-chemical characterization as well as pharmacokinetic studies. The in-silico docking and ADME prediction were also conducted to attain the knowledge about the novel pordrugs and their action in the central nervous system. In the present research work, use of prodrug concept, temporarily mask the acidic group of Flurbiprofen has been reveal as an innovative and novel approach to enhance the transport property across blood brain barrier (BBB). In this study, amide prodrugs of flurbiprofen with various amino acids such as phenylalanine, glycine and valine were synthesized by using Dicyclo hexyl carbodiimide (DCC) to obtain corresponding prodrugs such as Flurbiprofen-Phenylalanine (FP), Flurbiprofen-Glycine(FG) and Flurbiprofen-Valine (FV). The anticipated structures were confirmed by spectral analysis like IR, 1H NMR, 13C NMR and Mass spectroscopy. The hydrolytic studies were conducted to know the percentage release of the drug in various PH and that showed the considerable stability of the prodrug in the acidic environment. The in-silico docking study on beta-secretase enzyme as well as ADME prediction were done to understand the action of NSAID, flurbiprofen, in the activities of the brain. From the insilico studies, concluded that flurbiprofen having considerable BBB penetration ability and docking score in the beta secretase enzyme, which is having significant function in Alzheimer’s disease. So the amino acid –flurbiprofen prodrug approach is very beneficial and suggested for the degenerative conditions in the brain.
View:
- PDF (780.75 KB)