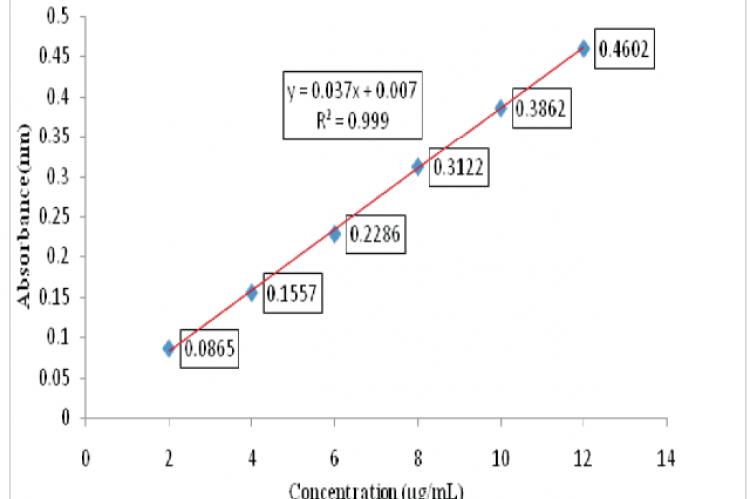
Worsening of asthma at night is commonly referred as nocturnal asthma (night-time asthma). It is believed that a rise in plasma histamine concentration at night causes nocturnal asthma. Leukotriene anatagonists are of specific use in the treatment of nocturnal asthma. Montelukast Sodium is an orally active compound that binds with high affinity and selectivity to the CysLT1 receptor. Chronotherapeutic Drug Delivery Systems refers to a treatment method in which in-vivo drug availability is timed to match circadian rhythms of disease in order to optimize therapeutic outcomes and minimize side effects. Considering the circadian rhythm of nocturnal Asthma, an attempt is made in the current study to develop a delayed-release tablet formulation of Montelukast Sodium as an approach of chronotherapeutic drug delivery system. A calibration curve was plotted at various concentrations of the drug substance. The observed results indicated a positive correlation (correlation coefficient R2 =0.999 and regressed equation y = 0.037x + 0.007) between concentration and absorbance. Optimized formulation was seal coated and enteric coated and evaluated for in-vitro drug release properties in 0.1M HCl followed by pH 6.8 Phosphate buffer and 0.1M HCl followed by 0.5% SDS solution. The drug release profile in 0.5% SDS aqueous solution differed from that in 6.8 pH phosphate buffer. Drug release started lately in 0.5% SDS solution compared to that in pH 6.8 phosphate buffer. However, drug release was relatively more in 0.5% SDS solution than in 6.8 pH phosphate buffer.
View:
- PDF (873.38 KB)