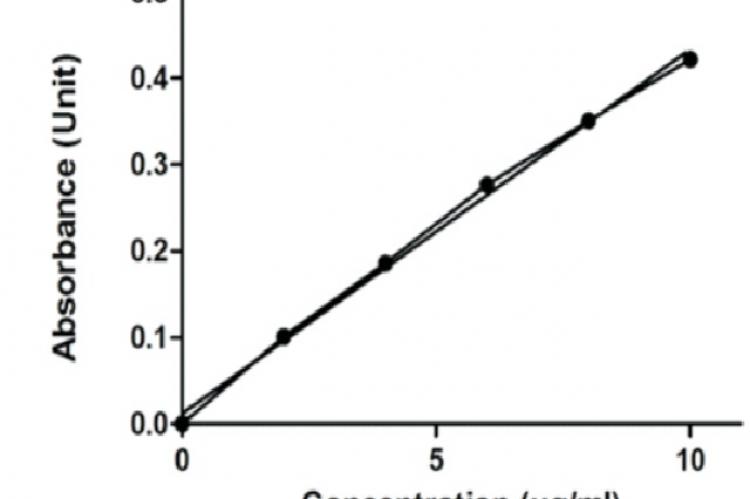
Purpose: A simple, precise, specific, and accurate High Performance Liquid Chromatographic (HPLC) and Ultraviolet (UV) spectrophotometer method was developed and validated for determination of Piroxicam (PRX) in pure and pharmaceutical dosage forms. Methods: The different analytical performance parameters such as linearity, accuracy, specificity, precision, and sensitivity (limit of detection and limit of quantification) were determined according to International Conference on Harmonization ICH Q2 (R1) guidelines. HPLC was conducted on Water Spherisorb® analytical column used having the dimension of 5 µm, 4.6*250mm. The mobile phase was consisting of buffer (containing 0.1 M potassium di hydrogen phosphate solution having pH 3.0) and acetonitrile (ACN) in the ratio 1:3 v/v, and the flow rate was maintained at 1.0 mL/min. PRX was monitored using Water Breeze 2 system equipped with photo diode array detector (λ = 333 nm) and also by UV spectrophotometer (λ = 333 nm) by Shimadzu UV 1800. Results: Linearity was observed in concentration range of 10–50 µg/mL by HPLC and 0-10 µg/mL by UV spectroscopy method. Correlation coefficient was found to be 0.9967 and 0.9962 respectively by HPLC and UV method. Linearity, accuracy, specificity, precision, and sensitivity (limit of detection and limit of quantification) were determined and value find within the range as specified by ICH guidelines. Conclusion: All the system suitability parameters were found within the range. The performed method is rapid, cost-effective and can be used as a quality-control tool for routine quantitative analysis of PRX in pure and pharmaceutical dosage forms.
View:
- PDF (1.78 MB)