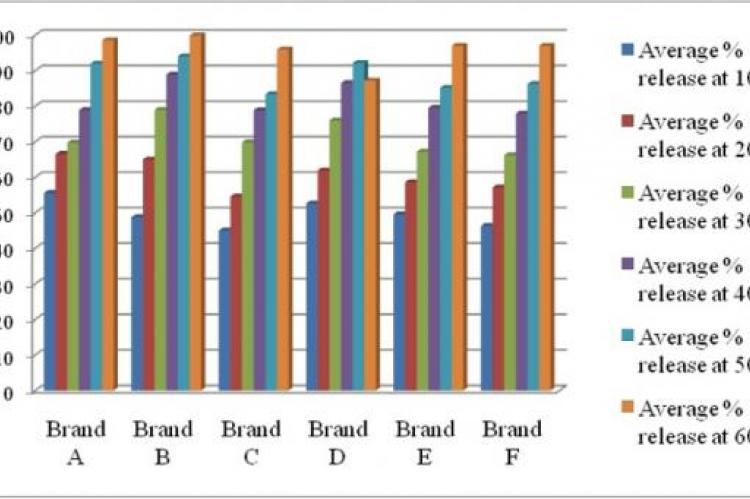
Choosing the proper drug product is getting complicated for health professionals and patients due to the existence of abundant generic brands in local drug market. The study was intended to evaluate the different physical parameters of generic amlodipine besylate tablet from different manufacturers using in vitro tests in order to minimize health risk factors and maximize the safety of local people. Six brands (A, B, C, D, E and F) of amlodipine besylate tablets (5 mg) marketed in Bangladesh were evaluated for eight in vitro tests including both official and unofficial viz. diameter test, thickness test, hardness test, friability test, uniformity of weight, disintegration test, dissolution test and assay. Dissolution study revealed brand B (99.87%) was the fastest and brand D (87.19%) was the slowest in terms of drug release. Using a validated UV spectrophotometric method assay value was recorded within 92% to 98.70%. Such study serves as a good pointer for assessment of in vitro parameters of commercially available products which may be advantageous for future formulation development studies.
View:
- PDF (878.3 KB)