Asian Journal of Pharmaceutical and Health Sciences,2014,4,4,1162-1166.
Published:November 2014
Type:Review Article
Authors:
Abstract:
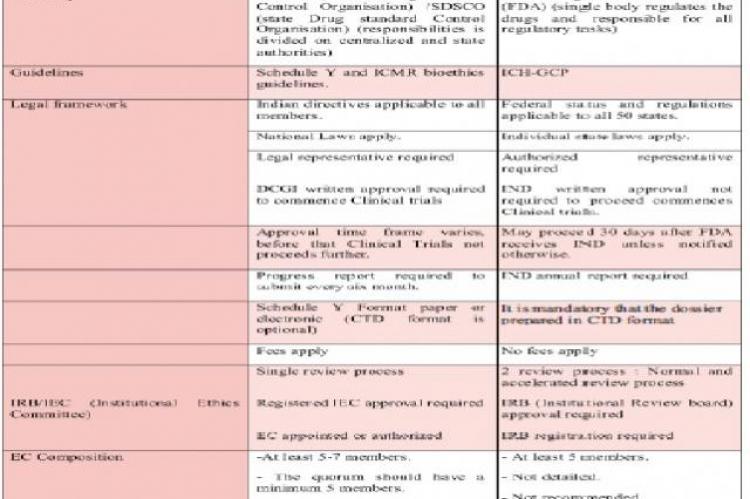
Presently the Indian industry is gripped with rising allegations of unethical trials and time-consuming regulatory approvals. Governing intellectual property protection regulatory processes are currently being updated to harmonize with United States and international standards, and plans are afoot to create a regulatory body in line with FDA, a central authority governing all drug development related activities. This article reviews the differences and similarities between present Indian regulatory board and the internationally acclaimed agency of Food and Drug Administration, United States.
View:
- PDF (1.43 MB)