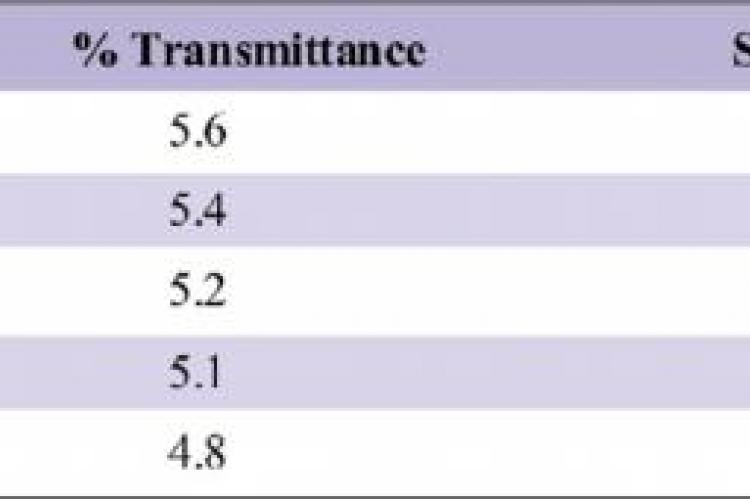
The aim of the study was to evaluate the quantity of 5% Sodium thiosulfate required to neutralize 0.5%, 1%, 2%, 3% and 5% Sodium hypochlorite.Different concentrations of the sodium hypochlorite (0.05%, 1%, 2.5%, 3% and 5%) were taken in different test tubes to which 5% of sodium thiosulphate, 0.1ml concentrated hydrochloride were added and 0.5ml crystal violet was added as an indicator. The volume of sodium thiosulphate was noted at which the crystal violet colour persisted. The volume of sodium thiosulphate was increased, if the crystal violet colour disappeared and this was done in fresh test tube repeating the same procedure. Though persistence of the colour itself indicated there was no excess of NaOCl left in the test tubes, all these solutions were decanted into the cuvette and cross checked by the spectrophotometric analysis respectively, by setting distilled water reference.1ml, 1.4ml, 2.4ml, and 3.5ml of 5% sodium thiosulfate when added to 1ml of 0.5%, 1%, 2%, 3% and 5% Sodium Hypochlorite respectively, the colour of the crystal violet persisted indicating there was no excess of hypochlorite left to decolourize it. The quantity of 5% Sodium Thiosulfate required to neutralize Sodium Hypochlorite of 2%, 3% and 5% is 1.4ml, 2.4ml and 3.5ml respectively.
View:
- PDF (169.28 KB)