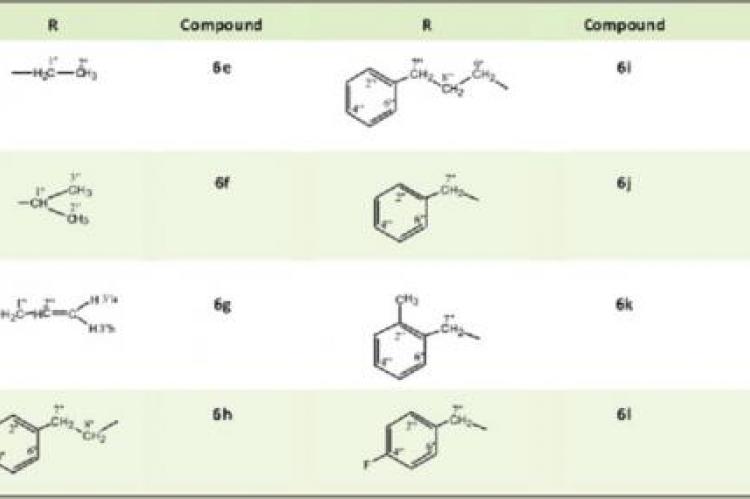
A series of S-substituted derivatives of 5-(4-nitrophenyl)-1,3,4- oxadiazole-2-thiol (6a-l) were synthesized in various steps. Organic acid 4-nitrobenzoic acid (1) was successfully converted into ester and consequently into its hydrazide in the presence of hydrazine hydrate and methanol as a solvent. Further, 4- nitrobenzoic acid hydrazide (3) yielded 5-(4-nitrophenyl)-1,3,4- oxadiazole-2-thiol, on treatment with carbon disulfide in the presence of base (KOH) and ethanol. Finally the target compounds (6a-l) were obtained by stirring 5-(4-nitrophenyl)- 1,3,4-oxadiazole-2-thiol with different electrophiles (5a-l) in the presence of sodium hydride (NaH) and dimethyl formamide (DMF). All these derivatives along with their parent compounds were characterized by IR, EI-MS and 1H-NMR spectra. These compounds were assayed for their antioxidant activities and other biological activities via screening them against acetylcholinesterase, butyrylcholinesterase and lipoxygenase enzymes, however, these showed prominent activity against acetylcholinesterase and butyrylcholinesterase enzymes.
View:
- PDF (690.48 KB)